Influence of potting mix properties on nutrient supply
- Mar 22, 2018
- 6 min read

In this third article published in Groundswell magazine on potting mix properties, Geoff Cresswell explains how the physical, chemical and biological properties of a potting mix influence plant nutrition.
Plants require 16 elements for healthy growth and to complete their reproductive cycle (Table 1). These essential elements, are divided into major and minor elements based on the amounts required. In addition, there are other elements that are considered beneficial to plant health including silicon, aluminium, cobalt, selenium and sodium. Of these, silicon is possibly the most helpful for plants grown in soilless media because they are generally low in plant available silicon. Silicon increases plant resistance to a range of pests, diseases and environmental stresses including moisture, temperature, wind and nutrient disorders.
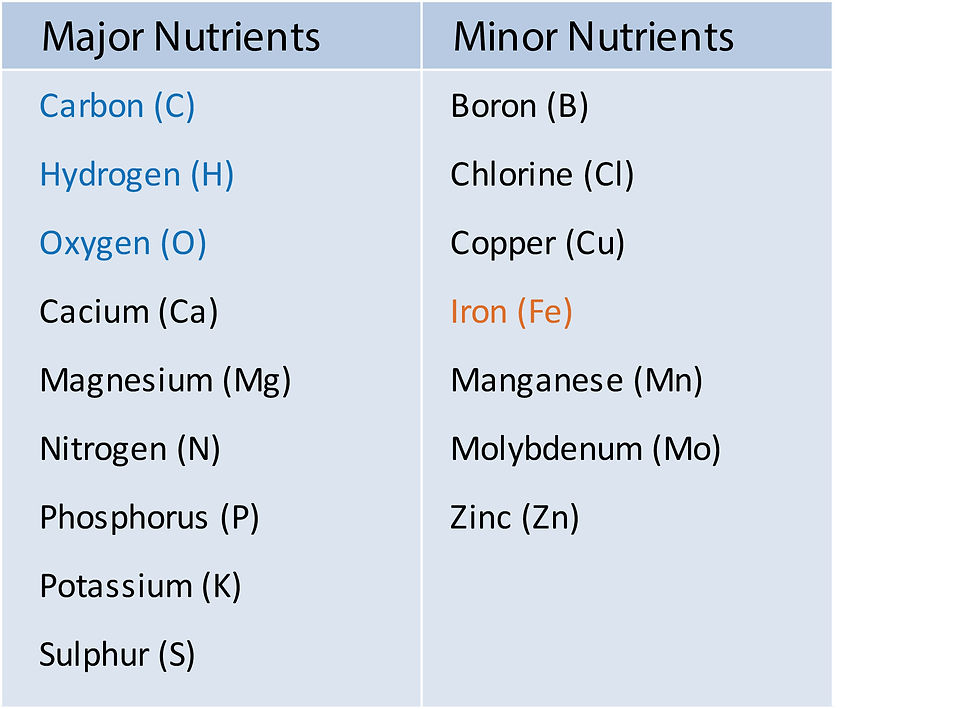
Table 1 Elements essential to plant health
No one essential element is more important to plant health than another. However, the supply of nitrogen normally limits growth most in horticultural systems. This is because plants require more of this element and because it is readily leached from potting mixes and consumed by microorganisms that decompose organic matter.
Plants absorb most nutrients from the soil solution through the roots. This means that the fertilizer must be either water soluble or readily broken down into ions by microbes to be plant available.
Solubility is important because nutrients must pass through a cell membrane in roots to enter the plant. This membrane is permeable to water but not to nutrient ions and most large organic molecules. If it was, the cell contents would leak out causing the cell to die.
The low cell membrane permeability prevents nutrient ions from freely moving into the cell at normal soil solution strengths. This property is essential because it allows plants to regulate the nutrients it absorbs through roots.
Uptake of nutrients across root cell membranes is controlled by electric pumps that are tuned to individual nutrient ions (Figure 1). These transporters are like key holes in the cell membrane formed by folded proteins. The holes have a shape and electrical charge that only allow ions with a specific size and charge (key shape) to pass through.
This micromachinery allows the plant to control the uptake of essential elements and to exclude elements that are potentially damaging such as sodium and chloride. It also enables nutrients to move into the plant against a concentration gradient (uphill).

Figure 1 Transport mechanisms for nutrients across the root cell membrane
Physical properties
Absorption through a transporter uses energy derived from respiration. This chemical reaction is fuelled by carbohydrate and requires oxygen. Roots get oxygen from the root zone which is why the air-filled porosity (AFP) of a potting mix is important.
Nutrient uptake is impaired when roots do not receive enough oxygen to fuel respiration. This is why plants in water-logged media develop nutrient deficiency symptoms. The main signs are pale green foliage and yellowing of the oldest leaves which are caused by nitrogen deficiency. Yellowing of new leaves which is caused by a trace element deficiency such as iron. Scorching and spotting of older leaves caused by the unregulated uptake of ions such as manganese and chloride.
Key points
The potting mix must supply enough oxygen to maintain active nutrient uptake. An AFP >15%v/v is adequate for most plants.
The potting mix must drain fast enough to prevent water-logging in wet weather.
Chemical properties
All nutrient ions have either a +ve or a –ve charge (Table 1) and so plants must excrete a counter ion (H+ or OH-) when the nutrient is absorbed to maintain a charge balance.

Table 1 Electrical charge on nutrient ions absorbed by plant roots
This balancing response alters the pH of the soil solution and this, in turn, influences nutrient availability (Figure 2). The optimum pH range for most plants (5.5-6.5) is where the trace elements are most available for uptake (except for molybdenum).

Figure 2 Influence of pH on the availability of plant nutrients
Because plants generally absorb more cations than anions, the pH of a potting mix usually falls over time. This change is accelerated when urea or ammonium is present in the fertilizer. Urea does not have a significant charge but is converted by soil microorganisms into ammonium and so behaves like a cation.
Not all plant species lower the pH (Figure 3).

Figure 3 pH of seedling mix after growing different plants under the same conditions https://www.ces.ncsu.edu/depts/hort/floriculture/plugs/ghsubfert.pdf
Nutrient supply is a function of the concentration of an element in the soil solution and how well the concentration can be maintained or buffered in the face of root uptake, leaching and competition from microorganisms.
Supply is also related to the available water content of a potting mix. Mixes that hold more water also hold more nutrient in reserve.
Cations resist leaching because they are held on negatively charged sites on surfaces. This property of a potting mix is referred to as the cation exchange capacity (CEC) and is mainly due to the presence of humified substances. These are products of organic matter decomposition and so CEC is increased by composting.
Modern potting mixes have relatively little anion exchange capacity. This means that nitrates, phosphates and sulphates are more susceptible to leaching than the cations. Anions will carry cations (usually calcium) with them in the drainage water and this accelerates acidification.
Buffering of nutrients can be improved by using slow release and controlled release solid fertilizers, by regular top dressing with soluble solid fertilizer, by liquid feeding and by increasing the CEC.
Key points
The pH of a potting mix should be less than 7 and greater than 5. Some plants can grow well outside of this range but most will not because the pH either reduces the availability of an element (iron) or increases it to the point where it becomes toxic (manganese).
Lime or dolomite should be added to a potting mix to counter acidification. Coarser grades are less susceptible to washing out of a potting mix.
The CEC of a potting mix should ideally be >100meq/L.
CEC can be increased by composting the organic components (pine bark, sawdust) and by adding products such as zeolite, diatomaceous earth or clay (vermiculite). Relatively large amounts of these additives are usually required to produce a significant increase in CEC and the cost may not justify the benefit.
Controlled or slow release fertilizers should be used to minimise leaching of the anions.
The EC of a potting mix should be <3dS/m (1:1.5v/v). Higher than this, and plant growth may be inhibited by osmotic stress particularly when the mix dries out between irrigation events.
Organic properties
Recent research indicates that plants can absorb organic forms of nitrogen such as amino acids, peptides and urea. However, this organic contribution is small relative to the inorganic forms of nutrients.
It now seems that plants may also:
Obtain some nitrogen by engulfing and consuming living and dead bacteria.
Release enzymes from roots into the rhizosphere to break down proteins into smaller molecules that are then absorbed to supply nitrogen. Some native plants excrete organic acids to dissolve soil minerals.
Absorb proteins into roots where they are degraded by enzymes to release nitrogen.
The organic properties of a potting mix can influence plant nutrition by reducing or by increasing the availability of nutrients.
Nitrogen drawdown is the process where microorganisms out compete plants for nitrogen dissolved in the soil solution. Drawdown is most severe in mixes that have not been adequately composted. Other nutrients (phosphorus and trace elements) can also be competitively removed this way by microorganisms.
Soil biology can also improve the availability of nutrients such as phosphorus and the trace elements. One way is by excreting organic molecules that act like chelating agents.
The physical properties of a potting mix will change with time due to the decomposition of the organic fraction which will reduce the particle size. This will in turn reduce the average pore size and so impact the air-filled porosity (lowered) and the water holding capacity (increased).
Potting mixes should be designed to perform well over the life of the crop which means that biologically stable materials should be used. Bark and sawdust should be composted as this improves a potting mixes stability and potentially provides a range of biological benefits to a crop such as disease suppression and stress tolerance.
Key points
Organic fertilisers need to be broken down by microorganisms to provide soluble nutrient forms that are available to plants.
All potting mix ingredients should be adequately composted to minimise nutrient drawdown, improve physical stability and to enhance the numbers of beneficial organisms.













Comments